PlexPCR® VHS
Detect and differentiate common lesion-causing pathogens
Herpes simplex virus (HSV-1&-2), Varicella zoster virus (VZV) & Treponema pallidum (Syphilis) in a single-well multiplex qPCR test
Simplify lesion diagnostics
Why use PlexPCR® VHS?
Syphilis rates are increasing around the globe and symptomatic or visual lesion diagnostic approaches may be missing some cases presenting as an atypical lesion. Molecular diagnostics are recommended for management of genital lesions to improve diagnostic accuracy and subsequent patient management.
- Ulcerations or lesions in the ano-genital and oral regions can be caused by a variety of bacterial and viral infectious agents.
- Syphilis lesions can present atypically, be painful, and appear indistinguishable from herpes.
- Up to 3% of genital lesions may be atypical zoster (VZV) presentations.
Validated on hundreds of clinical samples, PlexPCR® VHS demonstrates excellent sensitivity and specificity.

PlexPCR® VHS workflow solution
Streamline your workflow and increase productivity for rapid, routine diagnostics. Full automation options include sample and qPCR set-up, through to validated software solutions for automated result calling and simple data processing.
- Detect all four common lesion-causing targets or choose to detect herpes and varicella only.
- Validated on genital and non-genital samples
SpeeDx analysis software is included in contract pricing, is license free, and installed on a high security and GDPR compliant platform. Designed to support the IVD laboratory environment, features include:
- audit trails
- user traceability
- LIS connectivity
- QA and batch management
PlexPCR® VHS Performance Specifications
Demonstrated clinical performance – for more detail please see the PlexPCR® VHS Instructions for Use.
| Performance of PlexPCR® VHS | ||||
|---|---|---|---|---|
| HSV-1 | HSV-2 | VZV | T.pallidum | |
| Sensitivity | 97.7% | 100% | 100% | 100% |
| Specificity | 99.7% | 99.7% | 99.7% | 100% |
Specifications
The PlexPCR® VHS kit is a qualitative real-time PCR (qPCR) assay for the detection of Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2), Varicella zoster virus (VZV) and Treponema pallidum.
Targets
Single well: Herpes simplex virus 1 (HSV-1), Herpes simplex virus 2 (HSV-2), Varicella zoster virus (VZV), Treponema pallidum (Syphilis) & Internal Control
Sample types
- Swabs: genital, non-genital, anal/rectal and oral
Amplification instruments
- Applied Biosystems® 7500 Fast (7500 Fast)
- Roche LightCycler® 480 Instrument II (LC480 II)
- Bio-Rad CFX96™ IVD (CFX96 IVD)
- Bio-Rad CFX96™ CFX96 Touch™ (CFX96 Touch)
Shipping Conditions
Products are shipped on dry ice or ice gel packs.
Storage & stability
Expiry dates are stated on the labels. It is recommended that freeze/thaw cycles be limited to less than 15. Store protected from light at – 25°C to – 15°C.
Intended use
For in vitro diagnostic use. Not for sale in the USA.
Regulatory status
CE-IVD, TGA cleared, IVDR Certified
Resources
Please contact your local representative if you can’t find what you are looking for.
Information Brochures
Package Inserts
Safety Data Sheets
Current Software Version
- PlexPCR® VHS (7500) v3.1
- PlexPCR® VHS (LC480) v2.1
- PlexPCR® VHS (CFX) v2.1
- PlexPCR® HV (7500) v2.1
- PlexPCR® HV (LC480) v2.1
- PlexPCR® HV (CFX) v2.1
Related Products
Certificate of Analysis
For information contact [email protected]
Instructions for use
For information contact [email protected]
Software Download
For information contact [email protected]
Publications & Media
We are committed to supporting continued scientific discovery and generation of clinically relevant data. Collaborations with key researchers and clinicians around the globe help us improve diagnostics and address urgent clinical needs.

Multiplex PCR testing of anogenital lesions for Treponema pallidum and Herpes simplex virus in primary care improves the detection of Primary Syphilis.
Hughes, et. al. STI&HIV2023 World Congress, July 24 – 27, 2023 Chicago, USA.
Multiplex PCR testing for syphilis and herpes in primary care resulted in the detection of additional cases of primary syphilis. This is likely to have led to earlier diagnosis, treatment and potentially reduced infectiousness.
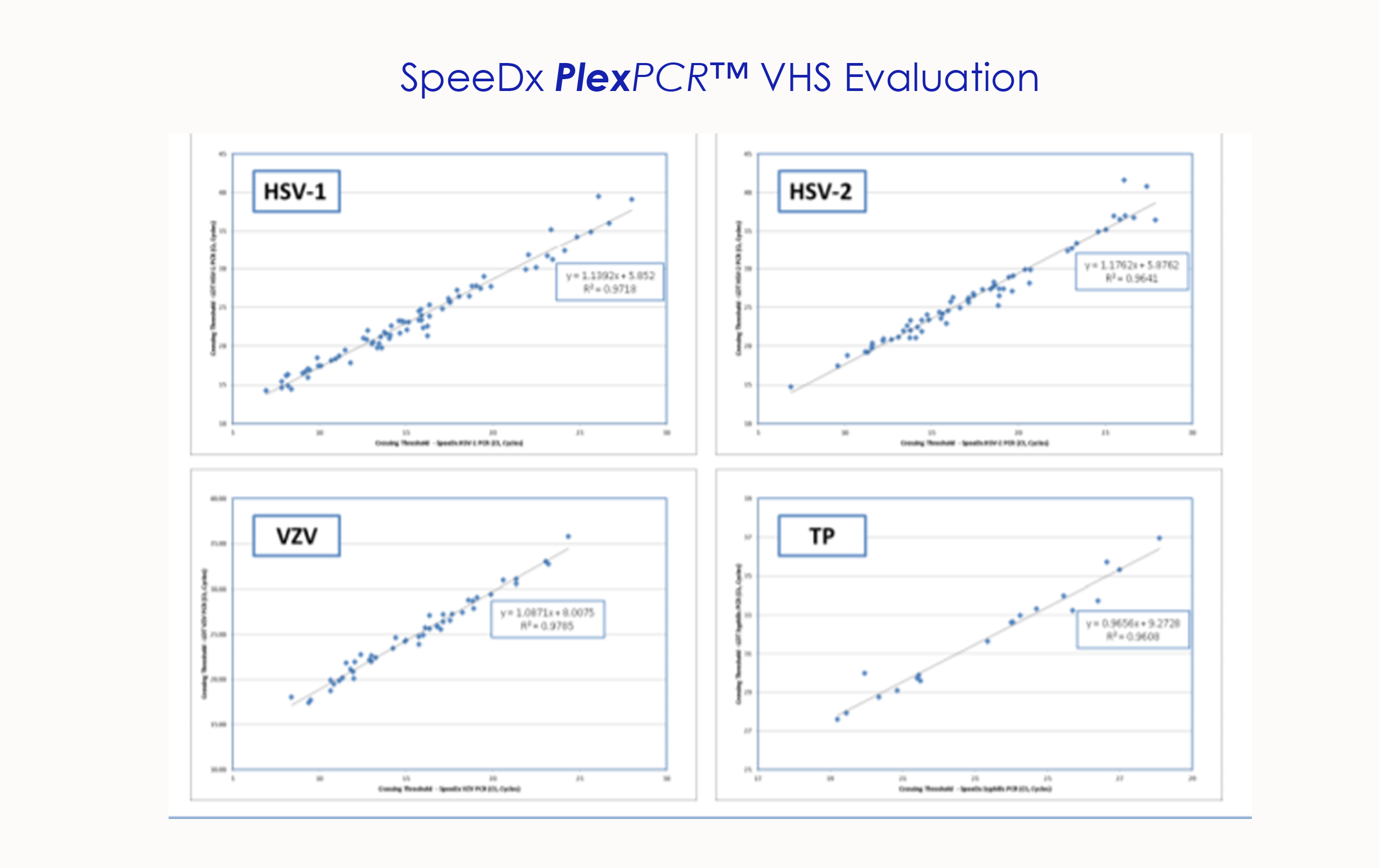
SPEEDX PLEXPCR™ VHS EVALUATION
Used PlexPCR® VHS to test 373 genital and non -genital swab samples compared to in-house method. Prevalence for each target: HSV-1 24%, HSV-2 21.3%, Syphilis 1.7% VZV 0.3%. Sensitivity and specificity of 96-100% across targets.

Performance characteristics of the SpeeDx PlexPCR® VHS assay for the molecular detection of herpes simplex virus, varicella zoster virus, and Treponema pallidum in lesion swabs
Goldstein EJ et al Diagn Microbiol Infect Dis. 2020; 99(2):115221
Used PlexPCR® to test 250 previously tested patient samples. The overall agreement between the PlexPCR® VHS and in-house assays was 97%. Negative agreement was ≥99%, and positive agreement for individual targets was 96% (47/49) for T. pallidum, 98% for HSV-1 and HSV-2 (50/51), and 100% (51/51) for VZV.

Performance characteristics of the PlexPCR® VHS assay for the detection of Treponema pallidum and other pathogens in genital and extragenital lesions
Qquellon-Palacios J et al. STI & HIV 2021 World Congress 14th-17th July.
Screened 72 genital and extragenital lesions with PlexPCR® VHS compared with conventional PCR for TP screening. Use of PlexPCR® VHS increased the detection of TP and other STI pathogens.
Ordering Information
Please contact SpeeDx for information about offering SpeeDx diagnostics in your clinic or for a list of current pathology clinics where SpeeDx testing is available.
| Product | Compatible | Size | Catalogue |
|---|---|---|---|
| PlexPCR® VHS* | LC480 II† | 100 reactions | 1121001 |
| ABI 7500 / ABI 7500 Fast / ABI 7500 Fast Dx҂ | 100 reactions | 1123001 | |
| CFX96 IVD/CFX96 Touch# | 100 reactions | 1125001 | |
| PlexPCR® VHS Analysis Software* | LC480 II† | 1 Unit | 99005 |
| ABI 7500 / ABI 7500 Fast / ABI 7500 Fast Dx |
1 Unit | 99004 | |
| CFX96 IVD/CFX96 Touch# | 1 Unit | 99006 | |
| PlexPCR® HV Analysis Software* | LC480 II† | 1 Unit | 99013 |
| ABI 7500 / ABI 7500 Fast / ABI 7500 Fast Dx |
1 Unit | 99007 | |
| CFX96 IVD/CFX96 Touch# | 1 Unit | 99014 | |
| PlexPCR® Colour Compensation*^ kit | LC480 II† | 2 reactions | 90001 |
*Not for sale in USA
҂Validated on the ABI 7500 Fast Instrument
†Validated on LightCycler 480 II
#Validated on CFX96 IVD
^Supplied upon request. This kit is only required for initial set-up to establish calibration of PlexPCR® and ResistancePlus® kits on the LC480 instrument, see instruction for use for details.