PlexPCR® RespiVirus
Detect 11 clinically relevant respiratory viruses
Influenza A, Influenza B, Rhinoviruses (A & B), Respiratory Syncytial Viruses (A & B), Human metapneumovirus, Adenoviruses, Human parainfluenza viruses 1, 2, 3 & 4 in a 2-well multiplex qPCR test
Increase productivity this respiratory virus season
Why use PlexPCR® RespiVirus?
Laboratories face increasing numbers of respiratory samples for testing each year as greater pressure is placed on clinicians to determine the cause of infection before prescribing antibiotics.
- Maintain clinically relevant turn-around times even during peak testing season.
- Ensure timely reporting of results to optimize treatment decisions for patient management.
Tested on hundreds of clinical and QAP samples, PlexPCR® RespiVirus demonstrates excellent sensitivity and specificity.


PlexPCR® RespiVirus workflow solution
Streamline your workflow and increase productivity for rapid, routine diagnostics. Validated software solutions included in contract pricing, is license free, providing automated result calling and simple data processing.
- Increase sample throughput compared with 3- or 4-well tests.
- Validated on a range of specimen types
- Flexible well configuration with 96 or 384 well options
- Run up to 1536 samples in under 7 hours with standard laboratory equipment
Option to fully automate your workflow with the PlexPrep™ liquid handling solution for sample prep and qPCR set-up:
- Compact system (63.5cm x 53.4cm, 38.5kg) with integrated touchscreen
- Compatible with 96- and 384-well plates
- Includes liquid level detection and monitored air displacement technology for accurate and reproducible performance
PlexPCR® RespiVirus Performance Specifications
Demonstrated clinical performance – for more detail please see the PlexPCR® RespiVirus Instructions for Use.
| Performance of PlexPCR® RespiVirus | |||||||
|---|---|---|---|---|---|---|---|
| FluA | FluB | RSV | RhV | HMPV | AdV | HPIV | |
| Sensitivity | 94.1% | 98.0% | 94.6% | 96.7% | 100% | 100% | 100% |
| Specificity | 99.2% | 99.7% | 99.4% | 96.6% | 98.8% | 99.7% | 99.6% |
Specifications
PlexPCR® RespiVirus is a qualitative, 2-well, one-step, reverse transcription real-time PCR (RT-qPCR) assay for the detection of Influenza A (FluA), Influenza B (FluB), Rhinoviruses (RhV), Respiratory Syncytial Viruses A and B (RSV A and B), Human metapneumovirus (HMPV), Adenoviruses B and C (AdV B and C) and Human parainfluenza viruses 1, 2, 3 and 4 (HPIV 1-4).
Targets
Well 1: Rhinovirus (RhV), Respiratory Syncytial Viruses (RSV) A and B, Influenza A (FluA), Influenza B (FluB), Internal Control
Well 2: Human metapneumovirus (HMPV), Adenoviruses B and C (AdV), Human parainfluenza viruses 1,2,3 and 4 (HPIV 1-4)
Sample types
- Swabs: nasopharyngeal swabs only
Amplification instruments
- Roche LightCycler® 480 Instrument II (LC480 II)
- Applied Biosystems® 7500 Fast Dx (7500 Fast Dx)
Shipping Conditions
Products are shipped on dry ice or ice gel packs.
Storage & stability
Expiry dates are stated on the labels. It is recommended that freeze/thaw cycles be limited to less than 15. Store protected from light at – 25°C to – 15°C.
Intended use
For in vitro diagnostic use. Not for sale in the USA.
Regulatory status
CE-IVD, TGA cleared, IVDR Certified, MoH Cleared
Resources
Please contact your local representative if you can’t find what you are looking for.
Safety Data Sheets
Current Software Version
- PlexPCR® RespiVirus (LC480) v2.0
Related Products
Certificate of Analysis
For information contact [email protected]
Instructions for use
For information contact [email protected]
Software Download
For information contact [email protected]
Publications & Media
We are committed to supporting continued scientific discovery and generation of clinically relevant data. Collaborations with key researchers and clinicians around the globe help us improve diagnostics and address urgent clinical needs.
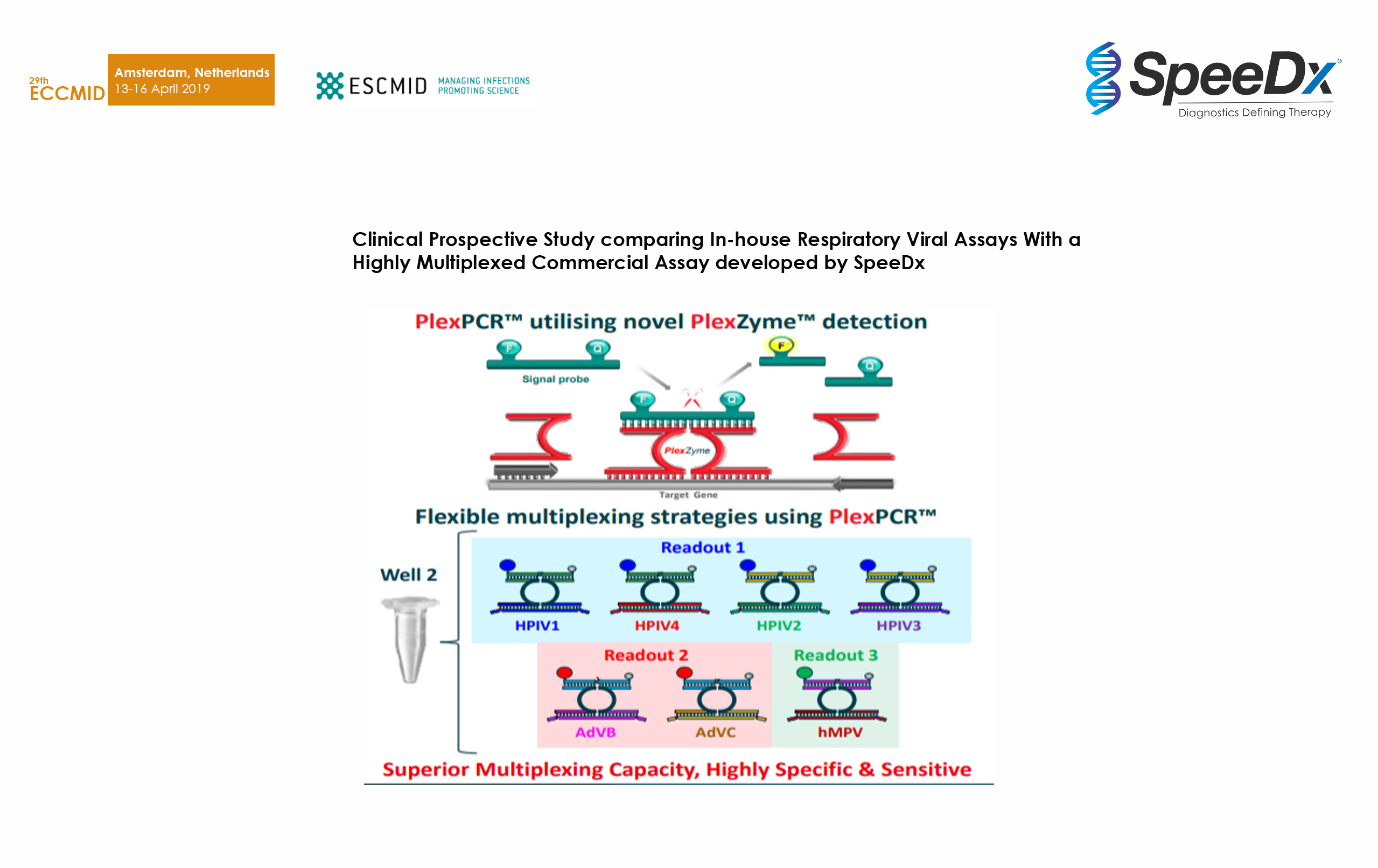
Clinical Prospective Study comparing In-house Respiratory Viral Assays With a Highly Multiplexed Commercial Assay developed by SpeeDx
Tested 204 upper respiratory and nasal swab specimens with PlexPCR RV 11 (beta) against in-house qPCR. Demonstrated high sensitivity and specificity even in complex multiplex assays with decreased reaction setup from 4 to 2 wells.

SpeeDx PlexPCR RespiVirus assay detects a broad range of contemporary human influenza strains and increases laboratory throughput and efficiency.
Bone S et al. 30th European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) accepted abstract April 2020.
Conducted in silico analysis to investigate the inclusivity of the PlexPCR® RespiVirus assay against contemporary human strains of influenza A and B viruses and conduct a workflow analysis to calculate sample throughput for a testing laboratory during respiratory virus season. All strains from 2018/2019 vaccine strains are expected to be detected. Workflow analysis revealed that a typical testing laboratory would be able to process up to 192 samples in 3 hours and 45 minutes.

Check back for more relevant papers and publications coming soon.
Ordering Information
Please contact SpeeDx for information about offering SpeeDx diagnostics in your clinic or for a list of current pathology clinics where SpeeDx testing is available.
| Product | Compatible | Size | Catalogue |
|---|---|---|---|
| PlexPCR® RespiVirus* | LC480 II† | 192 reactions | 1201192 |
| PlexPCR® RespiVirus Analysis Software* | LC480 II | 1 unit | 99011 |
| PlexPCR® Colour Compensation*^ kit | LC480 II | 2 reactions | 90001 |
| PlexPCR® RespiVirus Positive Control* kit | All platforms | 10 reactions | 95002 |
*Not for sale in USA
†Validated on LightCycler 480 II
^Supplied upon request. This kit is only required for initial set-up to establish calibration of PlexPCR® and ResistancePlus® kits on the LC480 instrument, see instruction for use for details.