ResistancePlus® MG
Detect Mycoplasma genitalium and macrolide resistance markers
MgPa for M. genitalium detection, plus four 23S rRNA mutations (A2058T, A2058C, A2058G, A2059G) in a single-well multiplex qPCR test
Enabling Resistance Guided Therapy
Why use ResistancePlus® MG?
Resistance to the macrolide treatment (Azithromycin) is of growing concern when managing M. genitalium (Mgen) infections. Guidelines around the world now call for the practice of Resistance Guided Therapy to guide appropriate treatment decisions, reduce potential for spreading infection among vulnerable populations, and support antibiotic stewardship.
- Resistance Guided Therapy is clinically demonstrated to improve patient cure rate and overall patient management.
- Detection of macrolide resistance can reduce time to cure, preventing ongoing transmission.
Tested on thousands of clinical samples, ResistancePlus® MG demonstrates excellent sensitivity and specificity.


ResistancePlus® MG workflow solution
Streamline your workflow and increase productivity for rapid, routine diagnostics. Full automation options include sample and qPCR set-up, through to validated software solutions for automated result calling and simple data processing.
- Validated on a range of specimen types and sample collection devices
- Validated on a range of sample extraction and qPCR platforms
SpeeDx analysis software is included in contract pricing, is license free, and installed on a high security and GDPR compliant platform. Designed to support the IVD laboratory environment, features include:
- audit trails
- user traceability
- LIS connectivity
- QA and batch management
Increase capacity of your existing qPCR instrumentation through universal run conditions compatible with all ResistancePlus® products.
ResistancePlus® MG Performance Specifications
Demonstrated clinical performance – for more detail please see the ResistancePlus® MG Instructions for Use.
| Performance of ResistancePlus® MG | |||
|---|---|---|---|
| MG detection | Resistance markers | ||
| Sensitivity | 98% | 92.5% | |
| Specificity | 100% | 100% | |
Specifications
ResistancePlus® MG simultaneously detects Mycoplasma genitalium and 4 mutations at positions 2058 and 2059 in the 23S rRNA gene (E. coli numbering) that are associated with resistance to azithromycin (macrolide-based antibiotic).
Targets
Single well: M. genitalium (MgPa), A2058T, A2058C, A2058G, A2059G, Internal control
Sample types
- Urine: Male and female
- Swabs: vaginal
- Pre-extracted samples
Amplification instruments
- LightCycler® 480 Instrument II (LC480 II, Roche)
- Applied Biosystems® 7500 Fast (7500 Fast)
- Applied Biosystems® 7500 Fast Dx (7500 Fast Dx)
- Bio-Rad CFX96™ IVD (CFX96 IVD)
- Bio-Rad CFX96™ CFX96 Touch™ (CFX96 Touch)
Shipping Conditions
Products are shipped on dry ice or ice gel packs.
Storage & stability
Expiry dates are stated on the labels. It is recommended that freeze/thaw cycles be limited to less than 15. Store protected from light at – 25°C to – 15°C.
Intended use
For in vitro diagnostic use. Not for sale in the USA.
Regulatory status
CE-IVD, TGA cleared, Health Canada cleared, IVDR Certified, MoH Cleared
Resources
Please contact your local representative if you can’t find what you are looking for.
Information Brochures
Package Inserts
- LC480 II – 100 pack size (PDF)
- LC480 II – 25 pack size (PDF)
- ABI 7500 – 100 pack size (PDF)
- ABI 7500 – 25 pack size (PDF)
- CFX96 – 100 pack size (PDF)
- CFX96 – 25 pack size (PDF)
Clinical Performance Studies
Safety Data Sheets
Current Software Version
- ResistancePlus® MG (LC480) v3.0
- ResistancePlus® MG (z480) v1.0
- ResistancePlus® MG (7500) v2.0
- ResistancePlus® MG (CFX) v1.0
- REFLEX ResistancePlus® MG (LC480) v1.0
- REFLEX ResistancePlus® MG (z480) v1.0
- REFLEX ResistancePlus® MG (7500) v1.0
- REFLEX ResistancePlus® MG (CFX) v1.1
Certificate of Analysis
For information contact [email protected]
Instructions for use
For information contact [email protected]
Software Download
For information contact [email protected]
Related Products
Publications & Media
We are committed to supporting continued scientific discovery and generation of clinically relevant data. Collaborations with key researchers and clinicians around the globe help us improve diagnostics and address urgent clinical needs.

Resistance-guided Antimicrobial Therapy Using Doxycycline-Moxifloxacin and doxycycline-2.5g Azithromycin for the Treatment of Mycoplasma Genitalium Infection: Efficacy and Tolerability
Durukan D et al. Clinical Infectious Diseases 2019 Oct 20;ciz1031. doi: 10.1093/cid/ciz1031.
Used ResistancePlus® MG to support Resistance Guided Therapy for Mgen infections, curing 92% and 95% of macrolide-resistant and macrolide-susceptible infections. Providing an evidence-base for current UK, Australian and European guidelines for the treatment of Mgen.
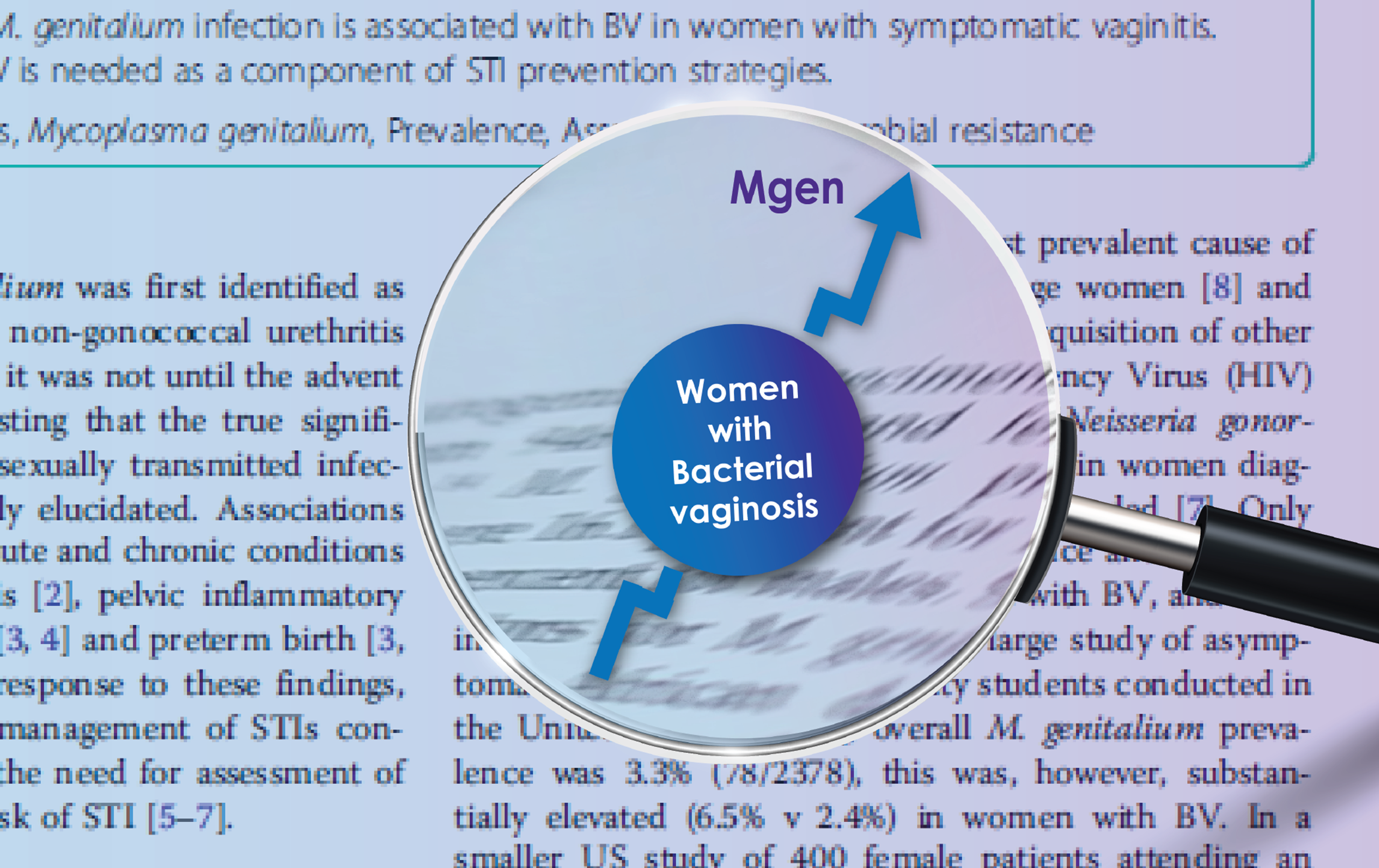
Prevalence of Mycoplasma genitalium Infection in Women With Bacterial Vaginosis
Nye M et al. BMC Womens Health 2020 Mar 26;20(1):62.
Used ResistancePlus® MG to investigate over 1500 symptomatic vaginitis patients. Mgen infection was more prevalent in women with bacterial vaginosis (BV) (7.0% Mgen positive) compared with non-BV vaginitis (3.6% Mgen positive). Macrolide resistance mediating mutations observed in over 37% of Mgen positive samples.

Mycoplasma genitalium – Management in the Era of Escalating Antimicrobial Resistance
Bradshaw C et al. SpeeDx Satellite Symposium (STI & HIV World Congress) July 12, 2017.
Results shared from the flagship Resistance Guided Therapy study demonstrating significant increases in cure rate of Mgen infections from less than 60% to over 92% using ResistancePlus® MG to identify macrolide sensitive and resistance infections to inform patient treatment.
Ordering Information
Please contact SpeeDx for information about offering SpeeDx diagnostics in your clinic or for a list of current pathology clinics where SpeeDx testing is available.
| Product | Compatible | Size | Catalogue |
|---|---|---|---|
| ResistancePlus® MG* | LC480 II† | 100 reactions | 20001L-01 |
| 25 reactions | 2000125 | ||
| ABI 7500 / ABI 7500 Fast / ABI 7500 Fast Dx҂ |
100 reactions | 2000201 | |
| 25 reactions | 2000225 | ||
| CFX96 IVD/CFX96 Touch# | 100 reactions | 2000301 | |
| 25 reactions | 2000325 | ||
| ResistancePlus® MG Analysis Software* | LC480 II | 1 unit | 99003 |
| ABI 7500 / ABI 7500 Fast / ABI 7500 Fast Dx |
1 unit | 99002 | |
| CFX96 IVD/CFX96 Touch | 1 unit | 99008 | |
| PlexPCR® Colour Compensation*^ kit | LC480 II | 2 reactions | 90001 |
| ResistancePlus® MG Positive Control* kit | All platforms | 10 reactions | 95001 |
*Not for sale in USA
†Validated on LightCycler 480 II
҂Validated on ABI 7500 Fast
#Validated on CFX96 IVD
^Supplied upon request. This kit is only required for initial set-up to establish calibration of PlexPCR® and ResistancePlus® kits on the LC480 instrument, see instruction for use for details.